Bearing Damage Index
Corrosion
Under normal conditions, bearing materials are not attacked by lubricating oils, but there are some adverse circumstances in which corrosion can occur.
Corrosion of the lead in copper-lead and lead-bronze alloys, and of lead-based babbitt, may be caused by acidic oil oxidation products formed in service, by ingress of water or coolant liquid into the lubricating oil, or by the decomposition of certain oil additives. Removal of overlays by abrasive wear or scoring by dirt exposes the underlying lead in copper-lead or lead-bronze bearings to attack, while in severe cases the overlays may be corroded.
Hydrogen sulphide in the oil attacks the copper in copper-containing alloys, including tin-based whitemetal. This hydrogen sulphide attack causes a dark deposit, mainly copper sulphide, on the surface of the bearing. It also causes depletion of the copper-tin compound in the lining, weakening the material.
Addressing the Damage
To find the cause of the corrosion, investigate the oil condition. A scanning electron microscope elemental analysis can identify any deposit on the bearing surface, providing insight into what oil contaminants may have caused it.
In the case of hydrogen sulphide attack, consider changing to a copper-free bearing specification.
AS40 (40% tin/aluminum) in place of tin-based babbitt has been used successfully to overcome problems of corrosive attack. Polymer-lined surfaces are also resistant to corrosion in all but the rarest of circumstances.

Figure 1: Corrosion of marine turbine whitemetal bearing; water in the oil has caused the formation of a smooth hard-black deposit of tin dioxide on the surface

Figure 2: Sulphur corrosion of phosphor bronze small end bush caused by decomposition of lubricating oil additive and gross pitting and attack of bearing surface
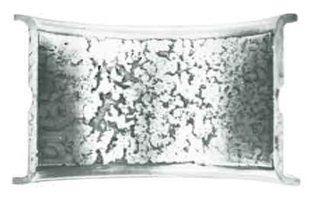
Figure 3: Severely corroded surface of unplated copper-lead lined bearing caused by the attack of lead phase by acidic oil oxidation products

